新潟大学工学部材料科学プログラム
新潟大学大学院自然科学研究科材料生産システム専攻
三俣研究室 Mitsumata laboratory
English pageACCESS
Staff

Dr. Tetsu Mitsumata
He received the PhD degree in Polymer Science in 1999 from Hokkaido University
under the direction of Prof. Y. Osada and Prof. J. P. Gong. After the PhD,
he studied stimuli-responsive polymer gels at Yamagata University as assistant
professor. In 2002, he joined the laboratory of Dr. P. Dekepper (CRPP Bordeaux
in France) to study nonlinear chemistry in solutions and gels. Now, He
works at the department of materials science and technology at Niigata
University as associate professor. He has published more than 70 scientific
papers, 17 review papers, 8 books, and 7 patents including international
patents, dedicated to Soft Materials, especially magnetic responsive gels
or elastomers. He has delivered invited lectures not only scientific conferences
but also manufacturing companies.
[Awards & Honors]
Encouragement award, Bridgestone soft material frontier prize (2010)
Best presented paper award, Chemical Evaluation and Research institute, Japan (2008)
Best presentation award, The Chemical Society of Japan (1999)
[Research & Project]
Viscoelastic response of magnetic soft materials
Stimulis responsive properties of synthetic and natural polymers
[Membership]
The Chemical Society of Japan
The Society of Polymer Science, Japan
The Materials Research Society of Japan
The Society of Rubber Science and Technology, Japan
[Selected Publications]
lonic state and chain conformation for aqueous solutions of supergiant
cyanobacterial polysaccharide, Phys. Rev., 87, 042607 (2013)
Magnetoelastic Behavior of Bimodal Magnetic Hydrogels Using Nonmagnetic
Partcles, Chem. Lett., 42, 50 (2013) Editor's choice
Magnetism and viscoelasticity of magnetic elastomers with wide range modulation
of dynamic modulus, Soft Matter, 9, 904 (2013)
Magnetic polyurethane elastomers with wide modulation of elasticity, Polym.
Chem., 2, 1063 (2011)
Thermosensitive solutions and gels consisting og Poly(vinyl alcohol) and
sodium silicate, Mater. Lett., 61, 3878 (2007)
pH-Response of Chitosan, k-Carrageenan, Carboxymethyl Cellulose Sodium
Salt Complex Hydrogels, Polymer, 44, 7103 (2003)
Solvent-Driven Chemical Motor, App. Phys. Lett., 73, 2366 (1998)
Reserch
- Variable Elasticity of Magnetic Soft Materials
We have succeeded to synthesize magnetic polyurethane elastomers and investigated the magnetorheological property, mechanical property, and degradation of the elastomers. The magnetic elastomer exhibited a reversible increase by facators of277 of the storage modulis and 96 of the loss modulus upon a magnetic field of 500 mT, which were nearly the same level with magnetic hydrogels demonstrating the giant magnetorheology. The magnetic elastomer maintained the magnetic field response for half year after the synthesis. In addition, the magnetic elastomer underwent high mechanical toughness with a breaking strain exceeding 0.8, and did not show a permanent deformation after removing the strain. These features clearly indicate that magnetic elastomers are suitable for materiale working under the air, than magnetic gels. We firmly believe that this magnetic elastomer which dramatically prolonged the lifetime will be widely used in the magnetically controllable smart devices in near future.



- Actuators Using Magnetic Soft Materials
We have succeede to fabricate a magnetic actuator consisting of magnetic polyurethane elastomere that demonstrate the elongation-contraction behavior by magnetic fields. The magnetic elastomer lifted up a weight of 10 kg with a stroke of 1.6 mm by applying a magnetic field of 300 mT. The time constant for the lifting was 200 ms. The maximum displacement of elongation was 4 mm without applying weights. We firmly believe that the high-power magnetic elastomer would be useful for the magnetically-driven actuator in practical use.
- Stmuli-Responsive Properties of Polysaccharides
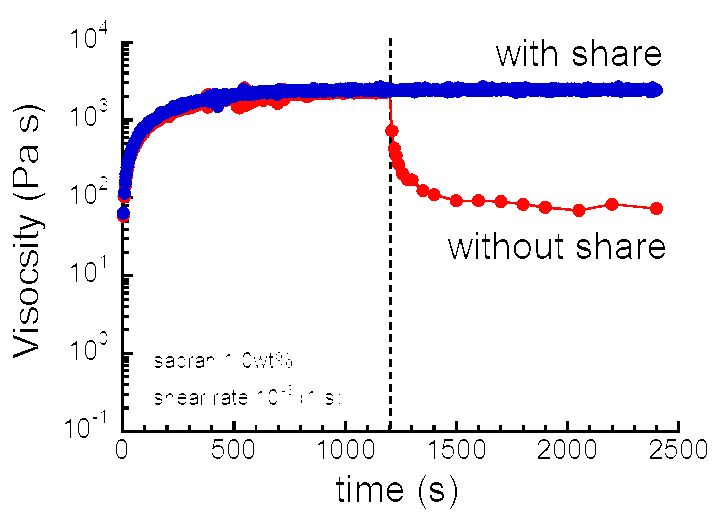
We have investigated the electric and rheological properties of the aqueous solution of cyanobacterial megamolecules named sacran. The molecular weight of sacran is 1.6×107 g/mol. The sacran is an anionic polyelectrolyte which has carboxylate and sulfate groups on the saccharide chain. Above the concentrations of 0.1 wt %, the sacran chain formed a weak gel which exhibits macroscopic liquid crystal domains. The sacran solution demonstrates shear induced viscosity when a constant flow with low shear rates was applied. When the flow is stopped, the viscosity is recovered to the original viscosity within 5 min. This phenomenon can be seen in other polysaccharides, however the increment in the viscosity for sacran aqueous solution is huge. We are investigating the mechanism of the negative thixotropy of the giant polysaccharide sacran.

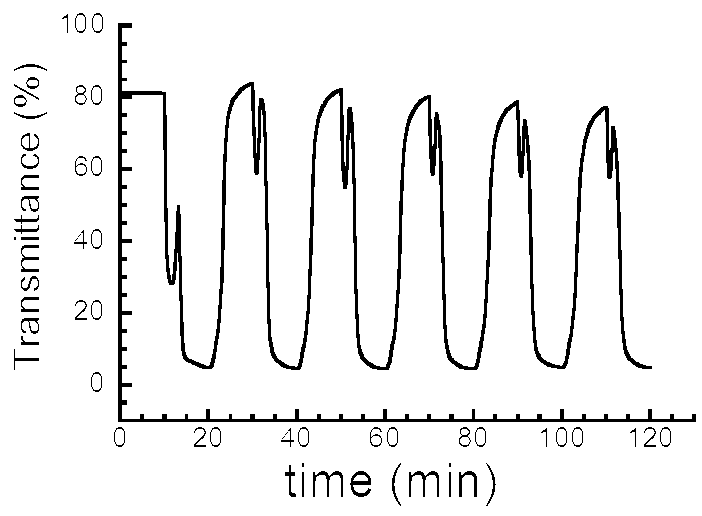
- Temperature-Responsive Properties of Composite Gels
We have investigated the transparency for visible light of a poly(vinyl alcohol) (PVA) aqueous solution in the presence of sodium silicates (SS). The transparency of the aqueous solution dramatically changed by varying temperature despite that neither aqueous solutions of PVA nor SS is sensitive to temperature. The aqueous solution showed a lower critical solution temperature (LCST) around 30 oC. The LCST depended on both concentrations of PVA and sodium silicate. Below the LCST, the transmittance was thermally irreversible. By a further increase in the temperature, the solution was separated into two phases consisting of a phase rich in solvent and that in solute; that is a coacervation. In the region of coacervation, the transmittance was thermally reversible. Reversible change in the transmittance was realized corresponding to stepwise temperature changes between 10 to 40 oC. A PVA gel swollen by the solution demonstrated transparency change in response to temperature changes as well as the solution.